Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts . Standard_electrode_potential_(data_pV pamätiPodobnéPreložiť túto stránkuThe data values of standard electrode potentials are given in the table below, in volts relative to. Redox equilibria of iron oxides in aqueous-based magnetite dispersions: Effect of pH and redox potential.
J. Colloid Interface Sci. 3(1): .

Oxidation-reduction potential refers to chemical reactions occurring in an aqueous solution called redox reactions or oxidation reduction reactions. Explains how to use redox potentials to predict the feasibility of reactions and select suitable oxidising or reducing agents. Just as we measure the activity of the hydrogen ion (a proton) by pH.
We measure the activity of electrons as pE.
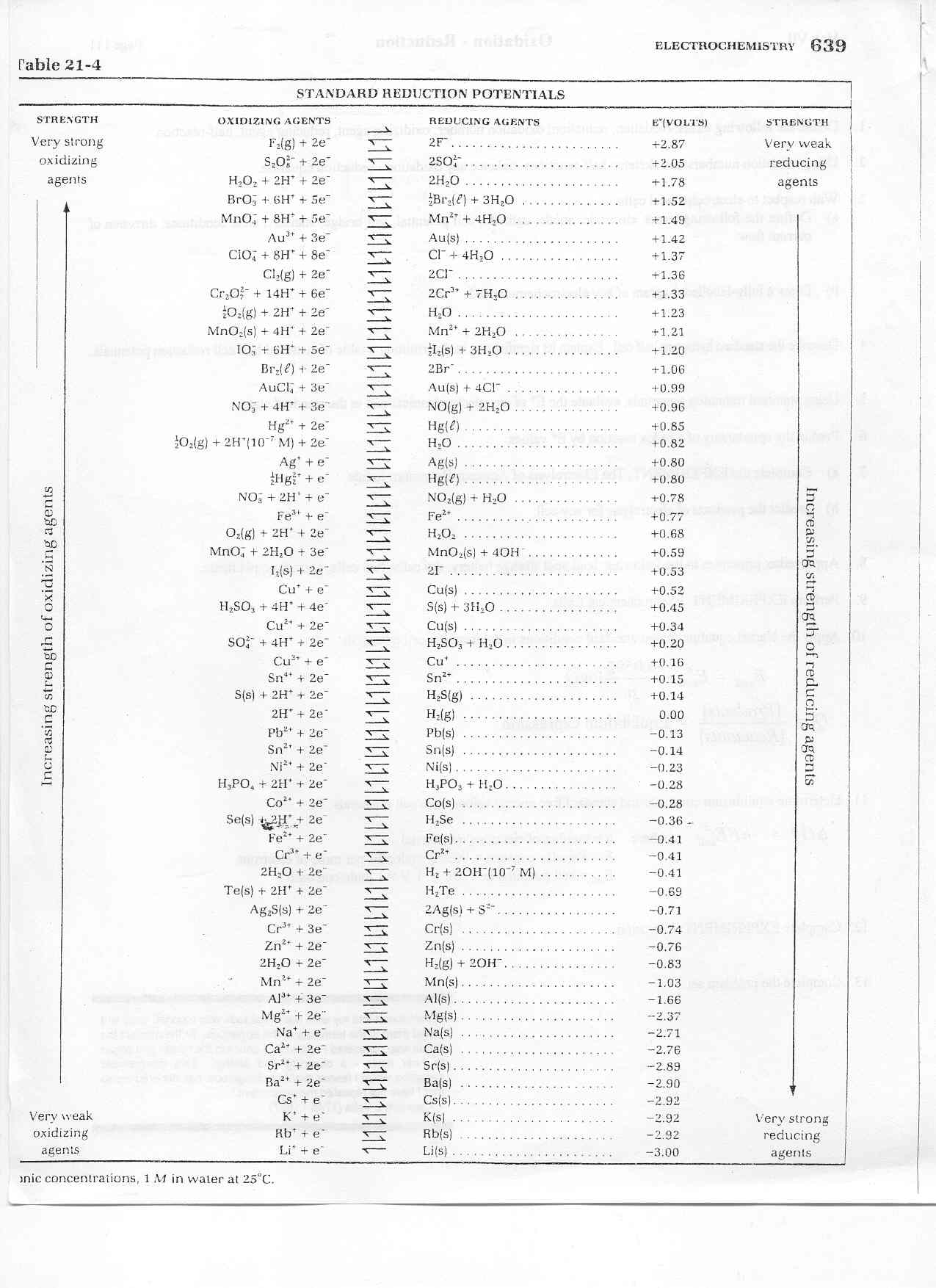
How to use a table of standard reduction potentials to calculate standard cell potential. Redox potentials are used to characterize the free energy cost and direction of reactions involving electron transfer, one of the most ubiquitous and important of . Standard Reduction Potentials in Aqueous Solution at 25oC. In oxidation-reduction (redox) reactions, the ability to donate or accept electrons is given by the redox potential, E.
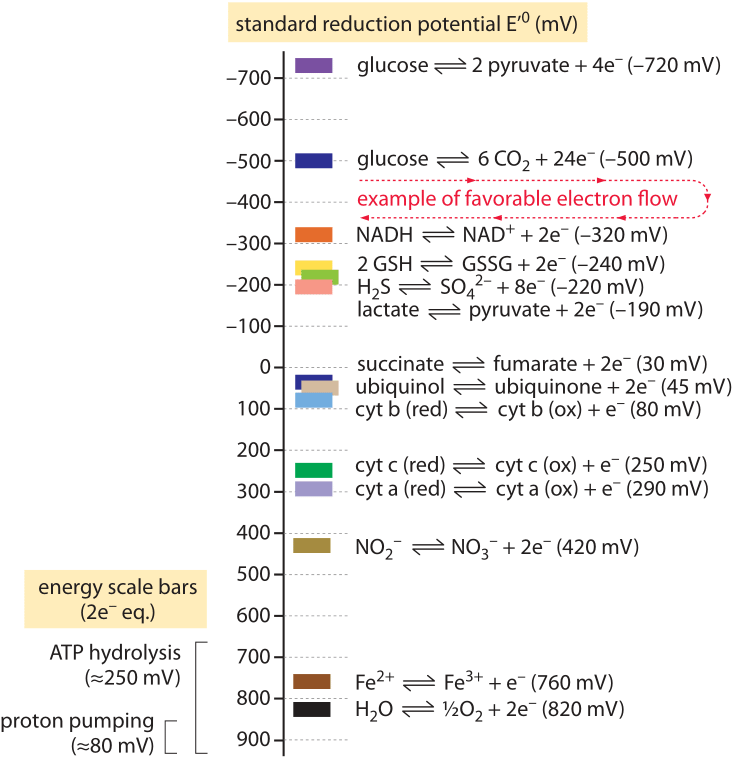
One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the oxidation-reduction potential or redox potential. Redox potential (also called oxidation-reduction potential or ORP) is an integrated measure of the balance between total oxidants and total reductants in a . Any oxidation-reduction (redox) reaction can be divided into two half reactions: one in which a chemical species undergoes oxidation and one in which another . The reactions which involve oxidation reduction are called redox reaction. In here HCl has been oxidised to Cland MnOhas been reduced to MnCl2.
Redox Potential Definition – Redox potential is defined as the specific indicator of the extent to which the oxidizing as well as reducing powers of a. Oxidation-Reduction Potential (ORP) or Redox potential measurements are used to monitor chemical reactions, to quantify ion activity, or to determine the . The redox potential (E or Eh) for non-standard conditions is measured with a calomel electrode or calculated with the Nernst equation. Current methods for determining ambient redox potential in cells are labor-intensive and generally require destruction of tissue. Redox potential as a master variable controlling pathways of metal reduction by Geobacter sulfurreducens.